



whole genome, one cell at a time
Explore Our Research
WHAT WE ASK
Embryos start out life as a single cell but quickly diversify into the bones, muscles, neurons, and skin that make up a plant or animal. How do these diverse cell types arise? Our lab is interested in how cohorts of genes orchestrate these developmental processes. In the process, we discover novel modes of genetic regulatory control.
HOW WE ASK IT
We address biological questions using cutting-edge technologies like single-cell-resolution genomics and single-molecule imaging. Our research combines hypothesis-driven molecular experimentation and computational analysis. Researchers in our group are trained in genetics, molecular biology, and computational biology.
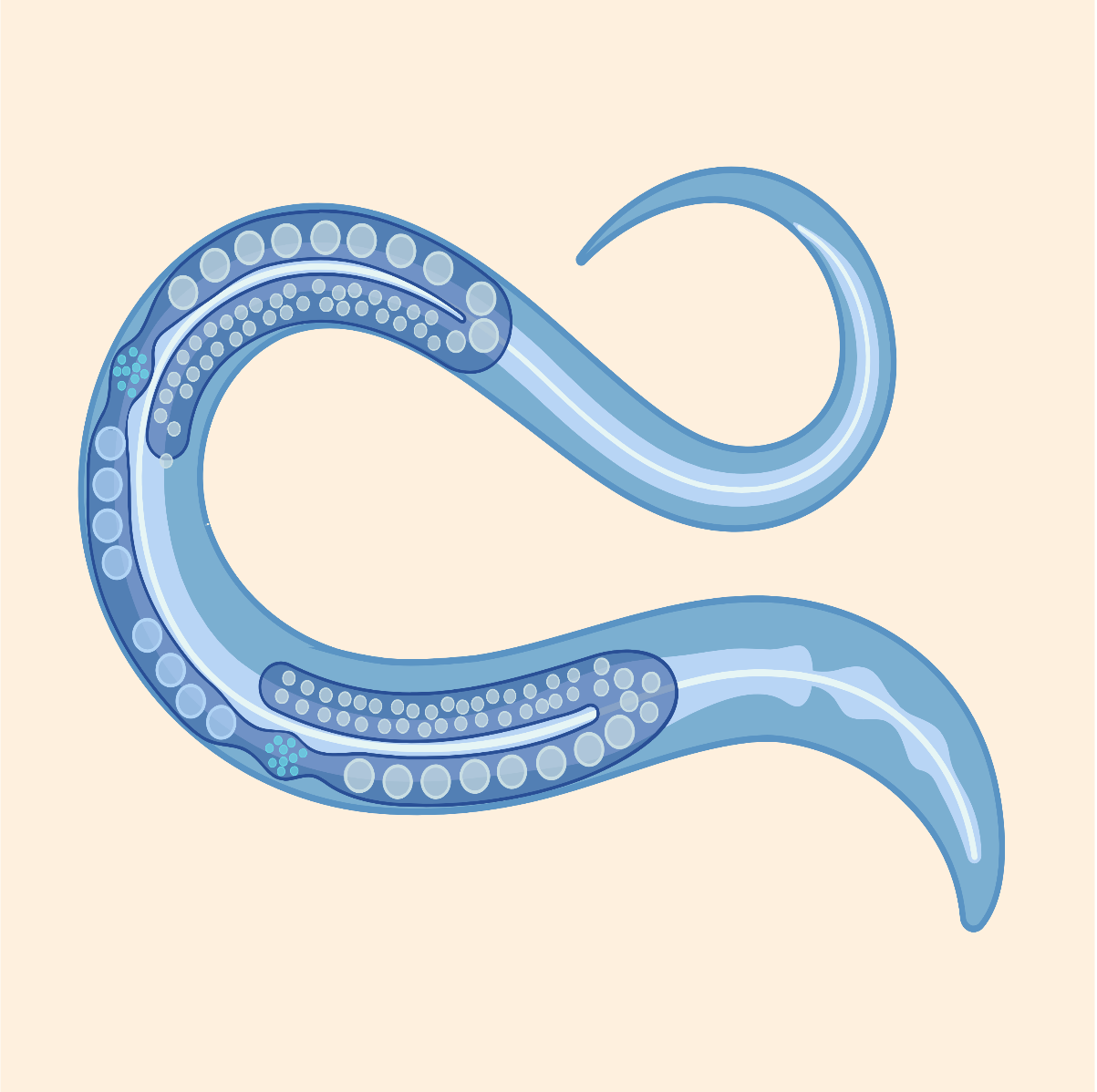
MEET THE WORMS
We use the microscopic nematode worm, Caenorhabditis elegans, as a genetic model. C. elegans are popular among researchers for their rapid and invariant embryonic development that can be viewed under the microscope over the course of a day. C. elegans were the first animal species to have their genome fully sequenced and are excellent for use in genome-wide studies. Furthermore, a large community of C. elegans researchers has worked together to develop resources that allow for precise genetic manipulation and experimentation. Because the characteristics and machinery of gene regulatory control are shared across animals, the things we learn in this worm model are relevant to human health.
Detailed Research Projects
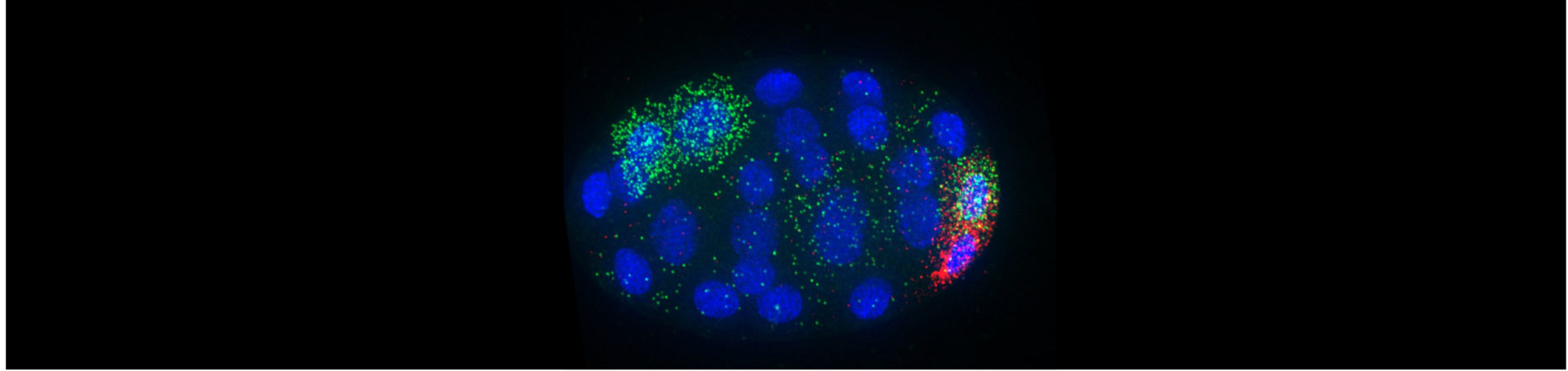
We have the ultimate goal of characterizing gene expression dynamics in developing C. elegans embryos. In an effort to characterize the differences in mRNA content between individual embryonic cells, Dr. Nishimura characterized the individual transcriptomes of the first two zygotic daughter cells using single-cell RNA-seq [Osborne Nishimura et al., 2015]. She later contributed to a collaborative effort to characterize the transcriptomes of all cells produced through the first four cell divisions [Tintori et al., 2016]. Interestingly, the transcriptomes of even the earliest C. elegans cells begin to diversify despite the fact that de novo transcription does not initiate at those stages.
How do differences in mRNA abundance arise? Our group hypothesized that post-transcriptional mechanisms such as differential mRNA stabilization, transport, inheritance, or decay drive the patterning. Interestingly, we have found that mRNA transcripts with cell-specific patterns of enrichment often accumulate in interesting regions within the cell! This was a big surprise. Our current research seeks out the mechanisms directing mRNA to concentrate in different regions of the cell. We also want to understand the reason why these transcripts accumulate in different locales
Our efforts led to recent insights into how and why mRNA transcripts concentrate into P granules (biomolecular condensates important for germline function. We found that transcripts move to P granules dependent on their translational repression [Parker et al., 2020] and directed by signals in their 3'UTR regions that impede translation, suggesting that P granules scavenge messages undergoing low to no translation. Current work in the lab is investigating mechanisms that direct mRNA localization to membranes.
All intestines digest food, but the intestine of the C. elegans nematode worm undertakes additional tasks that, in humans, are performed by separate organs. This includes coordinating fat storage, growth, genetic aging, environmental stress, and response to infection. At only 20-cells, the C. elegans intestine is a model of developmental genetics. In it, a network of GATA transcription factors directs intestinal cell fate by culminating in the expression of ELT-2 (Erythroid Like Transcription factor), the major intestinal regulator. This network has been instrumental in revealing general aspects of network biology, but critical gaps remain and we have yet to satisfyingly link it to the various tasks the intestine accomplishes once formed. Our research seeks to rectify unexplained aspects of the regulatory network and to expand the network to integrate stages of development when the intestine performs multiple functions.
Meet The Scientists

Kaelie Sellers
Undergraduate Researcher

Richard Bruno
Undergraduate Researcher

Alex Peirce
Undergraduate Researcher
William Coronado
Undergraduate Researcher
Lab Group Photos

Located at Colorado State University
in beautiful Colorado!